This week, a judge in Oklahoma ordered pharmaceutical company Johnson & Johnson to pay $572 million for its role in the opioid crisis that has ravaged the country and killed more than 6,000 people in Oklahoma alone. The ruling is the first to hold a drug manufacturer responsible for the crisis, which was fueled by companies flooding the market with addictive painkillers and pushing doctors to overprescribe the drugs. The amount is far less than the $17.5 billion that the state’s attorney general sought, and the company says it plans to appeal the ruling.
Cleveland County District Judge Thad Balkman ruled that the state met its burden in arguing that the company created a “temporary public nuisance” by using “misleading marketing and promotion of opioids,” and added in his ruling that “those actions annoyed, injured or endangered the comfort, repose, health or safety of Oklahomans.”
Judge Balkman cited Johnson & Johnson’s deceptive and aggressive marketing of painkillers to doctors, and the company’s practice of discouraging its sales representatives from discussing addiction or other negative consequences of using the drugs, while encouraging their prescription for both moderate and severe pain. The company also sought to convince doctors that they were under-prescribing pain medications and that having patients ask for higher doses was not a sign of addiction, just indicative of needing more to address their pain.
Johnson & Johnson markets the painkillers Duragesic (fentanyl) and Nucynta, both of which contain opioids. The company has also long manufactured the raw ingredients for other companies’ opioid-based painkillers, having bought a company in Tasmania in the 1980s that grows poppies and processed opium. According to the New York Times, by the height of the opioid epidemic, the company had become “the leading supplier for the ingredients in painkillers in the United States,” having developed a specific strain of poppy that provided the basis for Purdue’s Oxycontin, as well as manufacturing and supplying ingredients for “a range of other drugs, including hydrocodone, morphine, codeine and buprenorphine.”
Michael Ullmann, Johnson & Johnson’s general counsel, released a statement calling the judgement “a misapplication of public nuisance law that has already been rejected by judges in other states.” He also noted, “The unprecedented award for the state’s ‘abatement plan’ has sweeping ramifications for many industries and bears no relation to the company’s medicine or conduct.”
The amount decided for damages may actually seem low—$572 million will reportedly only fund a single year of Oklahoma’s opioid recovery plan, which the state estimates will cost $12.7 billion to $17.5 billion over 20 to 30 years. The company’s stock even rose this week, which some attribute to relief over the relatively low damages.
However, many are cheering the Oklahoma ruling as other lawsuits near their court dates. This includes a massive federal lawsuit scheduled for October in Cleveland, Ohio, that brings together more than 2,000 separate cases. Judge Balkman’s decision that the company’s activities constituted a public nuisance opens the door for similar rulings in other state cases, and an additional legal avenue for holding companies responsible for their part in the epidemic.
Also this week, Oxycontin manufacturer Purdue Pharma pledged to pay $10 billion to $12 billion to settle thousands opioid-related claims, according to NBC News. Purdue had been part of the Oklahoma suit, but to avoid the lawsuit, Purdue agreed in March to pay a $270 million settlement to establish an addiction treatment and research center at Oklahoma State University, and provide continued funding over five years. Purdue’s owners the Sackler family also agreed to pay $75 million to the center for five years. In May, Israel-based Teva Pharmaceuticals also settled with Oklahoma for $85 million, which will further fund the state’s effort to combat opioid addiction.

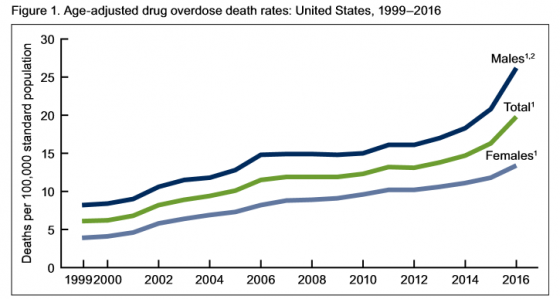

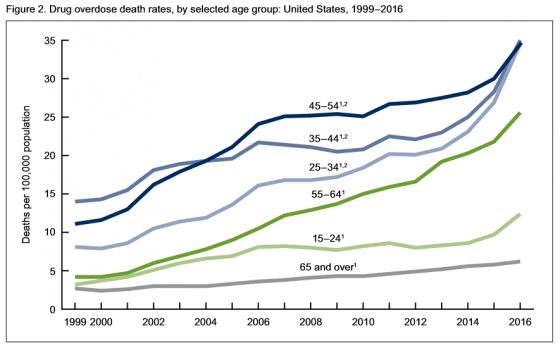
Authors of the report noted, “The pattern of drugs involved in drug overdose deaths has changed in recent years. The rate of drug overdose deaths involving synthetic opioids other than methadone (drugs such as fentanyl, fentanyl analogs, and tramadol) doubled in a single year from 3.1 per 100,000 in 2015 to 6.2 in 2016. Additionally, it’s important to note that many drug overdose deaths may involve multiple drugs.”
Of people age 15 and above:
• Rates of drug overdose deaths increased from 1999 to 2016 for all groups studied.
• Rates in 2016 were highest for people between the ages of 25 and 54.
• From 2015 to 2016, the drug overdose death rates for adults age 45-54, 55-64 and 65 and above went up 15%, 17% and 7% respectively, the CDC said.
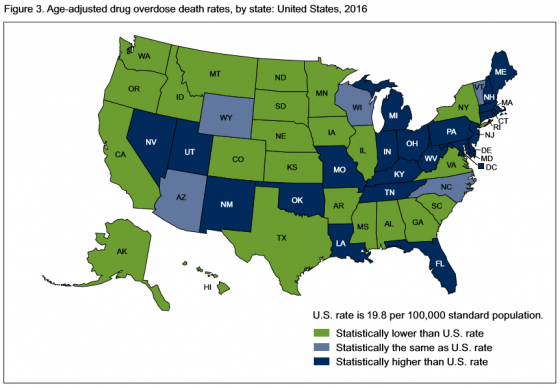
 In 2016, 22 states and the District of Columbia had overdose death rates that were statistically higher than the national rate. States with the highest number of overdose deaths were: West Virginia, with 52 per 100,000; Ohio with 39.1; New Hampshire with 39; District of Columbia with 38.8; and Pennsylvania, which had 37.9 deaths per 100,000.
In 2016, 22 states and the District of Columbia had overdose death rates that were statistically higher than the national rate. States with the highest number of overdose deaths were: West Virginia, with 52 per 100,000; Ohio with 39.1; New Hampshire with 39; District of Columbia with 38.8; and Pennsylvania, which had 37.9 deaths per 100,000.
States with the lowest age-adjusted drug overdose rates were: Iowa, which had 10.6 deaths; North Dakota, 10.6; Texas, 10; South Dakota, 8.4; and Nebraska, which had 6.4 deaths.
 In it’s most recent study, Quest Diagnostics found that workforce use of illicit drugs across the board—including cocaine, marijuana and methamphetamine—has climbed to the highest rate in 12 years.
In it’s most recent study, Quest Diagnostics found that workforce use of illicit drugs across the board—including cocaine, marijuana and methamphetamine—has climbed to the highest rate in 12 years.
Overall positivity in urine drug testing among the combined U.S. workforce in 2016 was 4.2%, a 5% relative increase over last year’s rate of 4%—the highest annual positivity rate since 2004 (4.
5%), according to an analysis of more than 10 million workforce drug test results.